Electromagnetic Radiation (EMR)
Table of Contents
What is the Electromagnetic Radiation?
Electromagnetic Radiation (EMR) or Electromagnetic energy is the energy propagated in the form of an advancing interaction between electric and magnetic fields. It travels with the velocity of light. Visible light, ultraviolet, and infrared rays, heat, radio waves, X-rays all are different forms of electromagnetic energy.
EMR ranges from gamma rays with very short wavelength to long radio waves. The shortest wavelengths can also be modeled as particles (photons). The interaction of EMR with matter forms the basis for Remote Sensing.
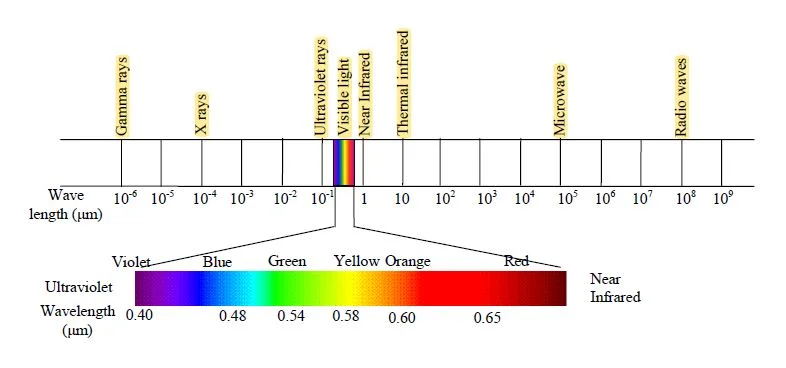
| UV | Visible(µm) | 0.8-0.9 (µm) | 0.9-1.3 (µm) | 1.3-14 (µm) | Microwaves | ||
| 0.4-0.5 | 0.5-0.6 | 0.6-0.7 | |||||
| Blue | Green | Red | Near IR | Mid IR | Far IR | ||
Electric and Magnetic fields that are perpendicular to each other and propagation to the direction of travel. Although it is a wave, it also can be detected as discrete particles of light, called photons.
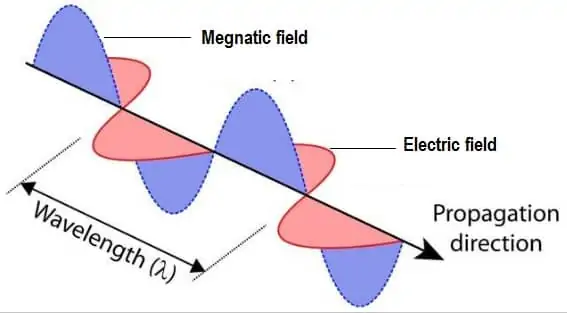
Electromagnetic Radiation Properties
- EMR radiated by atomic particles at the source (Sun)
- Propagates through the vacuum of space at the speed of light
- Interacts with the Earth’s atmosphere
- Interacts with the Earth’s surface
- Various optical systems and detectors
EMR Waves
| Waves | λ (m) | v (hertz[Hz]) | E (electron-volt [eV]) |
|---|---|---|---|
| Radio waves | 1 | 3☓108 | 1.24☓10-6 |
| Microwaves | 1☓10-3 | 3☓1011 | 1.24☓10-3 |
| Infrared | 0.75☓10-6 | 4☓1014 | 1.65 |
| Visible light | 0.4☓10-6 | 7.5☓1014 | 3.1 |
| Ultraviolet | 1.2☓10-8 | 2.4☓1016 | 1☓102 |
| X-ray | 1.4☓10-11 | 3☓1019 | 1.2☓105 |
| Gamma ray |
Radio Waves
Radio waves primarily come from the oscillation of currents in conductors. The long wavelengths travel easily and are not interfered by atoms (Except conductors).
Microwaves
The Microwaves are very short wavelength compare to radio waves. Microwaves are associated with the vibrations of some atoms and molecules. Water molecules act as a dipole.
Infrared Waves
Wavelengths longer than visible light are called Infrared rays. Molecules are heated by Infrared waves.
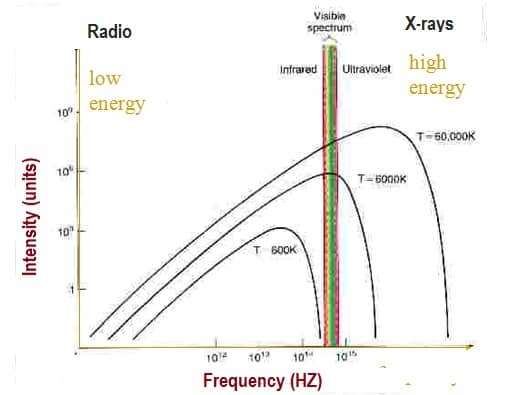
Visible Light
Visible Light is radiation in a narrow band of wavelength. Many atoms and molecules have specific behavior at unique frequencies of light. Corresponds to maximum output from the Sun.
Ultraviolet Waves
Ultraviolet rays have shorter wavelengths than visible light. Some molecules absorb ultraviolet and remit visible light.
X-Rays
X-rays are associated with energetic transitions in atoms. The short wavelength x-rays can penetrate materials.
Gamma Rays
Gamma rays are photons associated with nuclear or particle processes. Acceleration of a very energetic charged particle gives x-rays and gamma rays, it’s Called bremsstrahlung.
